17 years helping Australian businesses
choose better software
What Is Castor EDC?
Castor EDC is a comprehensive clinical research platform that enhances how clinical trials are run. It offers EDC, ePRO, and eConsent solutions, all aimed at improving data collection and clinical operations to accelerate the delivery of therapies, drugs, and devices to market safely. Castor prioritizes patient and site experience, integrating the human element into all aspects of its tech.
With experience in 15,000 studies with 8 million patients across 90 countries, Castor is adept at managing complex trials. Its cloud-native platform supports flexible deployment of modules for recruitment, data capture, & more, tailored to study requirements. Utilizing Practical AI and an API-first strategy, Castor simplifies the research process. Researchers can collect and analyze data from sources including clinics, homes, and wearables. Castor's flexible, robust technology advances clinical research accessibility and efficiency, promotes evidence-based medicine, and enhances global health.
Who Uses Castor EDC?
Who uses Castor EDC? Castor EDC, eConsent, and ePRO solutions serve 147,000+ users in the Medical Device, BioPharma, CRO, and academic sectors across 90+ countries, involving 8M+ enrolled patients.
Where can Castor EDC be deployed?
About the vendor
- Castor
- Founded in 2011
Castor EDC support
- Phone Support
Languages
Dutch, English
Castor EDC pricing
Starting Price:
- No free trial
- No free version
Castor EDC does not have a free version and does not offer a free trial.
About the vendor
- Castor
- Founded in 2011
Castor EDC support
- Phone Support
Languages
Dutch, English
Castor EDC videos and images
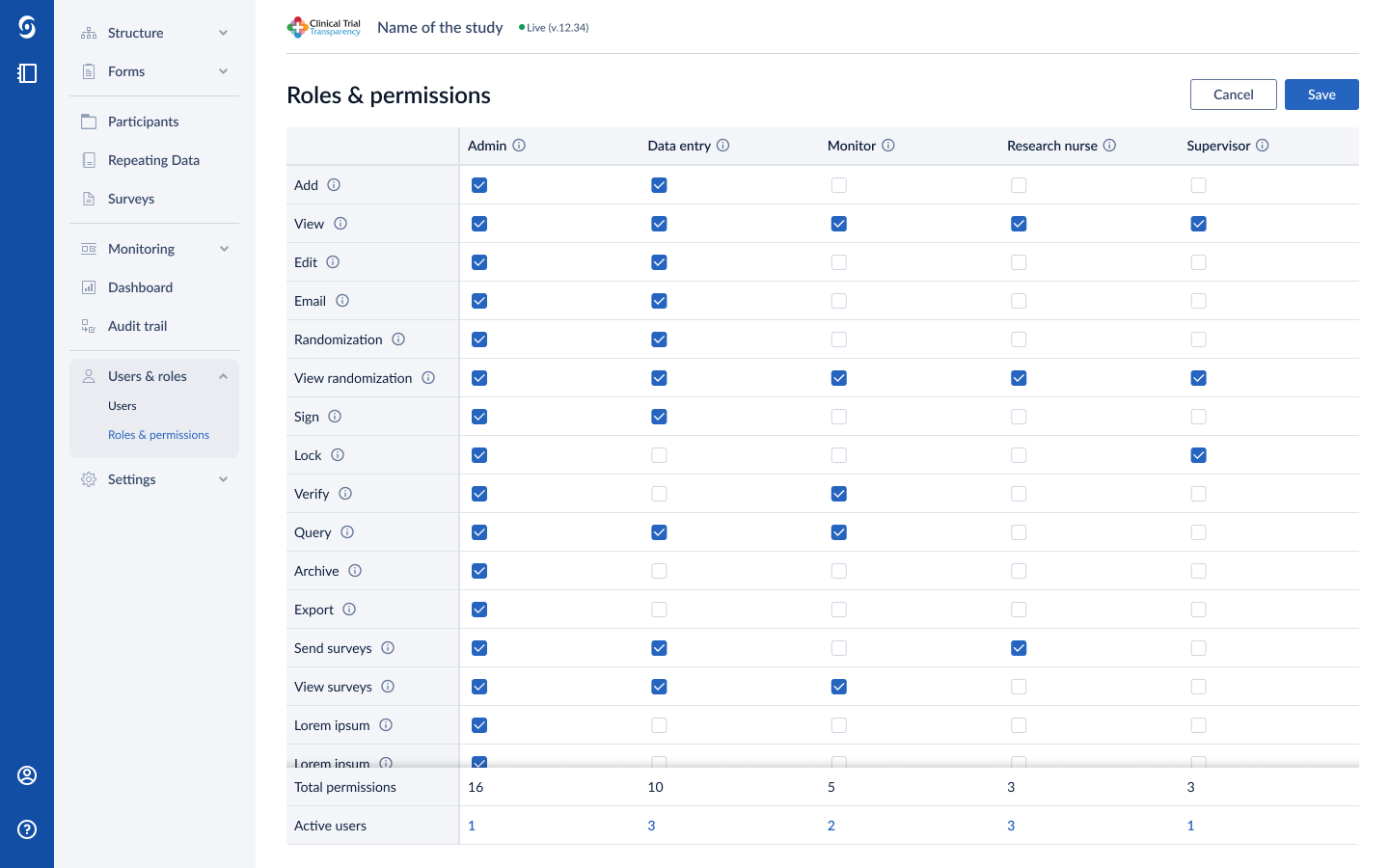


Features of Castor EDC
Reviews of Castor EDC

Good product, best service and easy to use
Pros:
The structure is intuïtive, solid and runs smoothly which is very important for multicenter research. It helped me significantly in my work as project manager of an international multicenter RCT.
Cons:
There are still options for improvement, such as a reminder for participating researchers of a coming deadline for a study visit (but Castor may have added this feature since I last used it).
Clear and easy to use
Comments:
My job is data entry in the context of clinical studies in various indications.
Finding and answering questions is very well structured.
Data entry is simple
I really enjoy working with Castor.
Pros:
I like the clear layout and the structure. This type of structure makes my work easier
Cons:
I have not found any disadvantages for my field of activity so far
Good, with lot of room for improvement
Pros:
The variability of options, as well as the way the system itself will correct the user when mistaken
Cons:
The rigidity and the lack of “delete options” in certain fields
Castor For Data Entry.
Comments: It's a great app and easy to use. This is my first job in data entry and Castor has made it easy.
Pros:
I like how smooth it is to switch to different tabs while working on data collection.
Cons:
I don't like the way dates are input to the data collection part of Castor.
Castor, what you need to manage your clinical trials
Comments: I really like working with Castor and has made the management of Clinical Trials easier.
Pros:
I really like about Castor that it has many different features that can be modified depending on the study, in a more specialized version. It is pretty useful also that all forms are exported together as one, required for the analysis.
Cons:
I am really happy with Castor right now.
Great Flow
Comments: I have worked in Castor in 10 different studies and never really encountered a problem
Pros:
Ease of moving from one subject to the next, as well as moving page to page within a subject.
Cons:
There is nothing about Castor that I do not like.
Great EDC system
Pros:
I find the CASTOR EDC very simple and easy to use. The updates that have been implemented haven't disrupted any data collection like some other systems do. The randomisation aspect is always quick and responsive, with no delay for the outcome. I find it to be an intuitive system.
Cons:
As mentioned, the randomisation aspect is quick, however since one of the updates the button has now moved to the left of the screen and it just says "not randomised". Until you select this option is may not be obvious that this is how you randomise patients as it looks like a statement, rather than an option to select. A minor fault in an otherwise great system!
Very helpful system for data collection in clinical trials
Comments: Very positive! Would highly recommend this system.
Pros:
- Clear - Easy to use - Track changes is automatic - Quick response from helpdesk - Easy data transfer
Cons:
- Sometimes the automatic track changes is a bit too sensitive: when I'm filling in the questionnaire and make 1 mistake, I have to change everything and explain it for every changed field.
Feedback about Castor
Pros:
Easy to use, built in a way that makes intuitive sense. Ample and timely feedback is provided
Great EDC performance!
Pros:
I like how the EDC looks because it is very visual, and it is easy to see what remains to be completed.
Cons:
Sometimes, the queries are not visible in which CRFs are located.
Castor EDC for academic research
Pros:
Easy to use, a lot of options for my academic research
Cons:
Had some few problems with navigating, however, after a few minutes, you get to know and its easy to use
CRA review
Pros:
User friendly, easy to use from investigator and CRA perspective.
Cons:
Site couldnt enter different units for lab results
Very usefull for questionnaire's
Comments: Very good, only minor things that were unclear for me. The instruction videos are very helpfull as a starter.
Pros:
It was very helpfull for sendig out a online questionnaire, very easy in use.
Cons:
Sometimes it's seems like there are multiple places for the introduction text and the only way to find out was to just trail and error.

Best Value and Also Best Product
Pros:
This software is intuitive to use. The company provides video training with all the ins and outs, even though you can work with it without the training. In the past, I had issues with obtaining a calculation for a specific formula and the customer support was so helpful, truly. Responses were almost immediate and my issue was resolved. In addition, the use of records and reports are so helpful when building the CRFs. This product includes EVERYTHING you can find in all of the competing products, including the regulatory requirements, but is so much less expensive that it is not even worth checking out the other companies. This is my go to platform for all of my clients' clinical trials.
Cons:
Watching the videos... I just want to hop in. I am writing this because I have to, honestly, there is nothing I don't like about this software.
Castor Response
5 years ago
Hi Sharon, Thank you so much for this super positive review and great to have you as a loyal customer! Our goal is to offer a product that covers all features necessary for any medical device or pharma study at a competitive price - so it's good to hear that it has indeed met your company's needs. Best regards, Elena Kochova, MD Customer Success Manager
Castor EDC - user friendly, with lots of options and a great helpdesk
Comments: Before they were using Castor EDC in this hospital, not everyone was working GCP-proof. With Castor EDC, they do. Benefits are that I can re-use some of the previously created eCRFs, which saves a lot of time and promotes standardization of databases.
Pros:
The software is user friendly, and it is clear that (medical) researchers were involved in the development of the software - which is very convenient. The software offers a lot of options, so users can design their own eCRF and databases. The manual offer a lot of information if you do not know you to create something you have in mind. Moreover, the helpdesk is Always very quick in responding to your questions. What I also appreciate is that Castor EDC is open for any suggestions for improvement.
Cons:
I do miss some options that I appreciated a lot while working with other similar software (in other hospitals). However, a pro is that I have mentioned those to the helpdesk and either they responded that they will incorporate them in future, or put them on a whish list, or they provided me a kind of work around. Unfortunately, not all the options I appreciated in the other software will be incorporated.
Castor Response
5 years ago
Dear Rosaline, Thank you for your review - we are indeed focused on compliance and data standardization, so thank you for pointing that out. We are developing Castor continuously so we hope that the features you are missing will be there soon! In the meantime, please do continue to give us feedback. Best, Elena
A reliable database for all types of studies
Comments: Dont let the cons bother you too much. Its a future proof software which every researcher should seriously consider using.
Pros:
+ Ease of use, it didnt take long to master it. + Castor EDC is supported by many companies as a reliable partner. + Castor keeps updating its product and services in order to keep up with developments in research law and good clinical practice. + No more paper! (Or rather.. less paper) + The ability to create case report forms of measurement instruments for future use in other studies. + Large amount of options for all types of studies.
Cons:
- The way the database is set up enforces the user to decide how he/she wants her data to be exported. - The system is not really designed for a high amount of measurements per step. It leads to some lagg in inserting data and it will nog let you make entire steps missing when applicable -> meaning you have to tag every single item as missing which is not very efficient. - The use of previously used/exported CRFs is still in development. For instance, only entire steps can be exported for future use though often steps contain multiple instruments or measurements.
Castor for clinical data management
Pros:
The amount of features for the data collection questions, several forms and options can be selected for different types of data. I have used many of the features to capture different data types. Also the options for randomization and surveys are very useful for clinical trials.
Cons:
For first time users it will be time consuming to make the data collection forms, however once you have the forms you can re-use them for new studies by importing the forms.
Castor Review
Comments: Very good! Since we use Castor our data quality has improved tremendously since our research staff (around the world) immediatly got familiar with Castor and found it much easier to use than the previous systems.
Pros:
How fast we could get started as Castor is very user friendly. We could build some eCRFs ourselves, and the support provided for the parts we could not do ourselves was very fast, adequate and high quality.
Cons:
Nothing so far. Some minor features that were not available were customly build for us when requested.
Castor Response
5 years ago
Hi Anne-Floor, Thank you for your all-star review! It makes us extremely happy to hear that you have found Castor so easy to use and valuable for your study! - Elena

Affordable EDC with Multifunctional Capabilities
Comments: We use CastorEDC for clinical trial data management. It's been great working with Castor, and their customer service always responds so promptly and is always super helpful.
Pros:
Easy data download process, great interface, and very affordable compared to the other EDC systems out there.
Cons:
There are still some minor bugs that the developers are fixing. Downloading large sets of data can be a bit slow and also delayed.
Castor Response
5 years ago
Hi Arick, Thank you very much for your positive review! User friendliness and customer support are our main focus points, so thank you for mentioning them. We wish you many successful studies in the future and please continue reporting bugs that you encounter :-) Best, Elena
Review of Castor
Pros:
- Ease of Programming. I don't have any programming experience, but I was able to build out the whole EDC database by myself, just watching the tutorial videos and explanations on the Castor website. - Responsive Support. Mainly interacted with Customer Support leads, but as a whole was impressed that the support team took our issues seriously and followed through to conclusion. - Low Price. Honestly, likely too low of a price for the value. Others in the field are something like 10x what you are charging, or far more. - User Interface. I like the changes made recently. Really opens up the screen.
Cons:
- Loading Time. This may be down to how I organized my Study with lots of repeated measures and a long architecture. But it takes a good 30-60 seconds to load up an individual Subject, and then 10 seconds or so to switch fields sometimes, most notably when I'm trying to dig through Reports. - Single Dependencies. Likely it would overcomplicate the programming, but being able to have more than 1 Dependency for an item would be useful. Only in a small percentage of cases, so the vast majority a single Dependency was sufficient. A like-to-have, not a need-to-have.
Castor EDC review
Pros:
The platform is really user-friendly for both the person who enter data and the data manager (in my case). We can quite easily build a study from the ground up or use some other studies as a template by importing the structure. The Monitoring tab is really helpful for the tracking of queries or warning messages.
Cons:
It is hard to tell what I do like least about Castor since I never used any other EDC system. I think that some features may be expanded. For instance, we have a cross-over study that will soon beon Live. We choose not to use the built-in randomization module because it was too limited for this specific study (it works really well for other studies). Also, the randomization tab is always available, which can lead to errors because some people randomize the patient before being certain that the patient can be included in the study.
Castor Response
4 years ago
Dear Cristophe, Thank you for your 5 star review! User friendliness is of course one of our main priorities! While some randomization features might be limited, we have recently introduced the possibility to use an external randomization list within Castor, so this might be helpful for some future studies! There are also workarounds for limiting the randomization to eligible records. Please reach out to our support team to discuss the options! Best regards, Elena Kochova, MD Sr. Customer Success Manager
Satisfied customer
Comments: 10 out of 10
Pros:
Very user friendly, very good customers service.
Cons:
At the time I used Castor, it was not possible to automatically send reminders to participants in case of a questionnaire wasn't filled in.
Castor EDC: growing and growing internationally
Pros:
Easy to use and understand. The manual provided is very extensive and answers practically all your answers. Researchers themselves can create their own database without extensive software knowledge such as Oracle Clinical. The helpdesk is also very swift in replying to one's questions.
Cons:
The study part of Castor is not yet compatible with the screens of mobile phones and tablets. Hopefully soon. The validations and dependencies could be extended to multiple fields to minimize the use of extra calculation fields.
Review Castor from monitor perspective
Pros:
Castor is very intuitive and easy to use. Per variable/step/phase I can verify the data. Also making queries is very nice, it has multiple steps in closing a query what makes it very clear. What's also great is that gaining access to a database is very simple and easily arranged.
Cons:
The audit trail cant be printed out, it can only be viewed online. But most of the times the audit trail is too massive anyway to print.
Great experience using Castor
Pros:
I had a great experience using Castor and will continue to use it with great pleasure in the future. Highly recommended to researchers for clinical trials.
Cons:
I have no cons regarding the software, very userfriendly