17 years helping Australian businesses
choose better software
What Is Researchmanager – Clinical Research Suite?
ResearchManager Clinical Research Suite is a centralized platform for clinical research, providing comprehensive access and control over every stage of a trial.
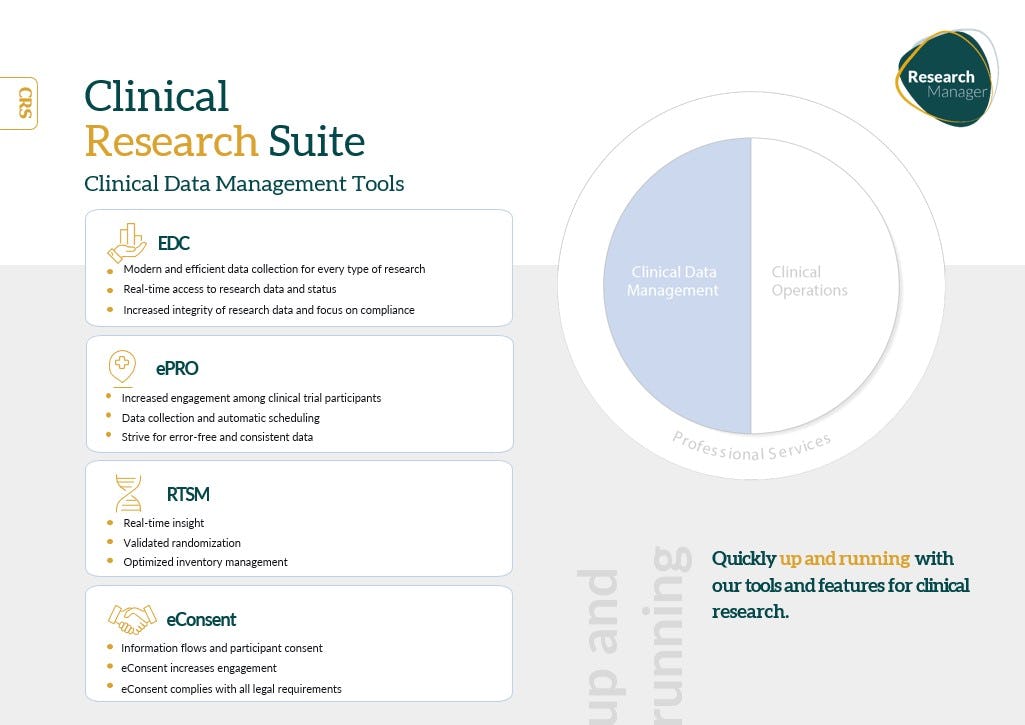
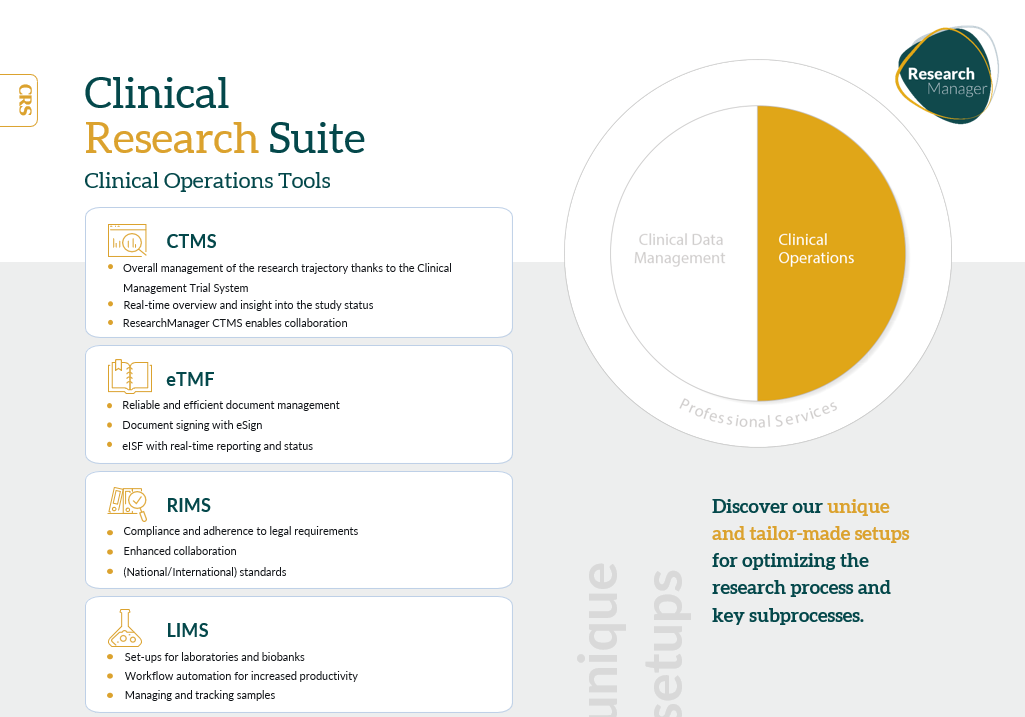
As a Software-as-a-Service (SaaS) solution, the Clinical Research Suite adapts seamlessly to the specific needs of clinical trials, offering a range of products for Data Management (EDC, ePRO, RTSM, eConsent) and Clinical Operations (CTMS, eTMF, LIMS, RIMS).
ResearchManager is compliant with ISO, GCP, GDPR, and HIPAA standards, operating from headquarters in the Netherlands and the USA.
Customers can choose between TIER3+ EU-based data centers and cloud solutions that offer regional data storage in locations like the USA, Canada, and Australia.
Choose the flexibility of the On Demand plan or scale with the Enterprise plan. Get a cost estimate within 1 minute on our website, and/or schedule a demo with one of our specialists.
Who Uses Researchmanager – Clinical Research Suite?
The Clinical Research Suite is utilized by CROs, academia, regulatory agencies, pharmaceutical, biotech, healthcare, and medical device companies.
Where can Researchmanager – Clinical Research Suite be deployed?
About the vendor
- Cloud9-Software
- Located in Deventer, Netherlands
- Founded in 2013
Researchmanager – Clinical Research Suite support
- Phone Support
- 24/7 (Live rep)
- Chat
Languages
Dutch, English, German
Researchmanager – Clinical Research Suite pricing
Starting Price:
- Yes, has free trial
- No free version
Researchmanager – Clinical Research Suite does not have a free version but does offer a free trial.
Pricing plans get a free trialAbout the vendor
- Cloud9-Software
- Located in Deventer, Netherlands
- Founded in 2013
Researchmanager – Clinical Research Suite support
- Phone Support
- 24/7 (Live rep)
- Chat
Languages
Dutch, English, German
Researchmanager – Clinical Research Suite videos and images




Features of Researchmanager – Clinical Research Suite
Reviews of Researchmanager – Clinical Research Suite
Excellent software
Pros:
Can exchange data with other research teams, even those working on projects at different trial sites.Provides the ability for electronic signatures, making it simpler to get informed approval from study participants and record it.
Cons:
Time consuming when transferring large data set from the application
How I use ResearchManager
Comments: I started using the research management to imput some patients information, it let me work remotely with other centers.
Pros:
How easy is to imput the information, it really helps me to look and review what other researchers in my project upload.
Cons:
The company I work with in the USA gave me the oportunity to use it and since the project started I haven't had any issue
Using research manager for getting committee approval
Pros:
Support quickly replied and forwarded me to the correct person to solve my problem.
Cons:
International researchers need to work with the research manager for getting committee approvals, since it only supports the dutch language the work with the research manger is very complicated. Also the open field, which need to be filled during the submit-process are badly explained. The process is not streamlined and although the Amsterdam UMC policy is to let outsiders file submits on their own, the fields do not support submission with non-Amsterdam UMC mail addresses.
Proces and Document Management System
Comments: It does what it's meant for
Pros:
Organizes an essential process of protocol routing
Cons:
Not all buttons are totally intiutive. It requires some training and experience.
New user
Pros:
it's easy to use, in my experience access the data and put the data on the document is very easy, everyone could adapt to his own necessities
Cons:
some data must be calculated in an easy way
Makes setting up a study easier
Pros:
At my University, we have been using this software for the past few months to set up a research project and it is a significant improvement than we had before. It is easy to use and it makes the process faster than before.
Cons:
I did not notice any downsides yet, I have been using it only as of recently.
Miskolc review
Pros:
That fact that I can see how the data was filled in for a previously involved patient.
Cons:
That I do not have a written Data Entry guideline.
Overall Good
Comments: Good Experience with lot of learning
Pros:
Designing and data entry is faster than other edc
Cons:
Have some issue with the site adding in the serial number.
metc Isala
Comments:
The best bennefit is the cloud based all in one system for submitting and reviewing any type of study .
The system is easily accessible and supports all parties in the assessment of a study. In addition, the system has an archif function where all documents are saved. The approved and the rejected. The minutes and questions from the committee members remain transparent to all parties at all times. If a printout has to be made for overviews of studies or study components, this system is supporting this. You can sort on every detail of/in the application. This saves the secretariat and its staff a lot of time.It is easy to make overviews on request for investigators or for the competent authority when in need.
Pros:
it is easy for investigators to upload their study. They can follow the progress of the descission making process.... The METC has all the docs/letters/review questions/and meeting information in 1 application. The members of the METC have easy acces to the application and they can add their review from any laptop/computer or phone from anywher in the world as long as there is internet.
Cons:
It some times can be a bit slow , that can be frustrating!
review research manager
Comments: we use it for data collection for scientific studies
Pros:
- easy in use - many possibilities - good option to use one database with multiple accounts, even from different companies or hospitals
Cons:
i have no cons about the software, we use it a lot
Mostly great
Comments: I am mostly pleased
Pros:
It's mostly very easy to use. I have never had any seriously negative experiences with the software.
Cons:
Sometimes not always the most intuitive.
Cloud9-Software Response
2 years ago
Dear Xaviera, Thank you for taking the time to post a review. Glad to hear you like our system! Thierry ResearchManager
User friendly
Comments: I used it for my research were participants filled out a very long questionnaire. It was easily converted to SPSS in just a few seconds. It's a real time-saver!
Pros:
It's a very user friendly program once you get to know it.
Cons:
Sometimes you can't 'code' things beforehand, luckily you can change it very easily in SPSS so it wasn't a problem
Top program
Pros:
Easy to use program that maken it the best for me
Cons:
Nothing, I really like the program and use it often
Easy to use
Pros:
Easy and understandable. Great to be able to export the data to SPSS
Cons:
No option to select multiple participants to mark as 'missing'. Would save time!
Find my way
Pros:
Everything is on one place, so centralised.
Cons:
I had to find my way, ask a few times and now i know its easier
evaluation
Comments: good
Pros:
user friendly good oversight of all items
Cons:
lot of clicking not easy loading of documents
Research Manager
Comments: Overall experience is good, sending documents to the EC is easier now.
Pros:
It is easier to submit an protocol to the EC
Cons:
Give every document a letter + number is very annoying..
Just a great software!
Comments: Very handy to keep an overview, easy to use. Always can look back at you documents.
Pros:
Very easy to use. Great lay out, so you can find your documents etc. easily.
Cons:
Nothing I didn't like or would improve.
Great Product
Pros:
I work for an orthopedic practice that collaborates with different medical device companies. Previously our work was paper-based but with RM we have been able to create and collect data in an efficient way. This platform is very user-friendly, with real-time data capturing and meets all the Good Clinical Practice and confidentiality criteria required in our practice.
Cons:
There is no real-time troubleshooting option available. If the issues issnt addressed in the FAQ an email needs to be sent and in most of the cases the response is in dutch.
It's a good program, easy to use. I wish the technical support department was more responsive.
Comments: It's a good program, easy to use. I wish the technical support department was more responsive.
Pros:
that you can easily edit it yourself, I like that as an administrator I can have several studies connected
Cons:
sometimes the system is not intuitive and you have to look for some tabs
Cloud9-Software Response
2 years ago
Dear Malgorzata, Thank you for taking the time to post a review. Glad to hear that our system is flexible. We take your points for improvement seriously for continuous improvements. Thanks for that! Thierry ResearchManager
Review WCN - LoDoCo2 trial
Comments: The helpdesk is not very keen on providing quick responses on more difficult questions.
Pros:
The software is easy to use and has very good export options to work with the data during the trial.
Cons:
That the monitor options are not so well defined. The query module is very counter intuitive and the pages can only be locked after many steps. The same holds for the monitor option.
Easy to use
Pros:
Efficient and Fast data save automatically.
Cons:
Overall good but for finding all patient data list we need to go to home task.
Cloud9-Software Response
2 years ago
Dear Nagma, Thank you for your positive review! We really appreciate it. Thierry ResearchManager
User shortcoming point
Comments:
Its a pitty you can not overwrite some documents.
I mean, for some documents there are templates in Research Manager. But as we have to undersign these documents, we can not overwrite these documents. You have to add a new document instead of overwriting the template!
Pros:
Its easy in use and clear! One can see in one blink of an eye which documents are missing.
Cons:
There is not much I dont least about this software! Just the 1 point I describe here under
overall outstandin application.
Comments: there is no problem while using research manager.
Pros:
how the way research manager working on data capturing that is very nice. easily assess.
Cons:
capturing all the data quickly. overall very good application
Review by a research nurse
Comments: It is easy to use and you can use Study Manager as a project management system which is really helpful and efficient if used by the whole company.
Pros:
Easy to use: data manager is user friendly for data entry. Sudy manager gives a clear overview of the trial.
Cons:
Lack of some functionalities, e.g. I switch a lot from 'Self Service > Invitations' to 'CRF Data' to check the level of the reported data by a subject. There are a lot of clicks involved, it would be easier if you could go directly from a subject number to the CRF data. Also, the program is a bit slow, e.g. to select a site and a subject number.